The Causes of Carpet Color Loss
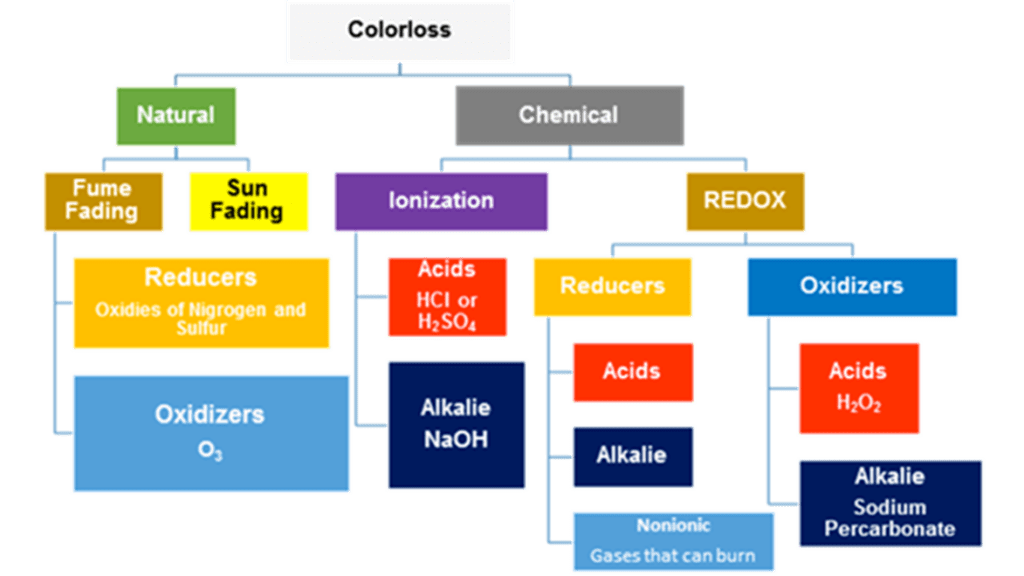
Color loss may be caused byelements in nature such as ultraviolet rays from the sun or atmospheric gases. These are normally referred to as natural color losses. Other types of color losses are referred to as chemical color losses.
Either way, they are both related to chemistry, or more specifically, chemical reactions.
Chemical reactions
There are two types of chemical reactions: reduction/oxidation (REDOX) and Ionization
- REDOX reactions, which are either reducers from sodium hydrosulfite or from others substances. Or, it is from oxidizers, such as from peroxides.
- Ionization of which there are acids, such as hydrochloric or sulfuric, which will destroy nylon, but not wool. They will also show up as strong reducing agents. Hydrochloric will show a high chlorine content. Alkalies come from concentrated hydroxides, such as what is found in oven and concrete cleaners. Many of these will show up as oxidizers.
Order in which the dyes fade
Generally, color loss happens one primary hue at a time, especially for natural color loss. The general order is for blue to go first, followed by red and then yellow. This means that a brown carpet will first turn orange, then yellow and finally white.
There are specific substances that destroy only one color. For example,
- Formaldehyde destroys red dye. Formaldehyde is in wooden floor products.
- Freon destroys yellow dye. Freon was the common coolant used in air-conditioning.
Measuring for chemical reactions
REDOX and ionization can be measured on meters. REDOX can be measured on an oxidation reduction potential (ORP) meter. The negative numbers indicate a reducer and the positive values indicate an oxidizer.
- Mild reducers for removing coffee and tea have ORP values of approximately of -200
- Strong red dye stain removers have ORP values of less than -450
- 3% hydrogen peroxide has an ORP value of approximately 360.
- Strong peroxide stain removers have ORP values of 450 and more.
Natural color loss
Natural color loss will not have a contrast in pH values compared to the rest of the carpet. Generally, there is nothing to neutralize.
- Sun fading is a form of oxidation and will happen near windows, especially on the southwest exposure of a dwelling. In as little as six months, all the blue could be missing from the exposed areas.
- Fume fading can occur either as a form of reduction or oxidation.
- Reduction will generally have a splotchy appearance. It comes from substances called oxides of sulfur or nitrogen. Both of these gases would be associated with oil refineries, and they both smell.
- Oxidation comes from ozone, O3, which generally occurs in drier climates with a lot of motorized vehicles.
Chemical color losses
Chemical color losses will generally have a pH that is more than 0.3 difference from the surrounding carpet. The pH and sometimes the ORP values will need to be neutralized before new dye can be reapplied.
The best neutralizers for alkali pH values is acetic acids; the best neutralizer for acidic residues is ammonium hydroxide. That is because both are volatile. However, other forms of mild acids or alkali can work too. However, you may want to avoid ammonium hydroxide when chlorine is in the carpet; it could make a poisonous gas. Chlorine can be measured using a chlorine meter which looks and operates similar to the pH and ORP meters.
Color loss is common to both nylon and wool carpet. Generally, both are dyed with acid dyes and have problems with alkalinity, especially wool.
Nylon
Nylon 6 is more prone to loss color compared to Nylon 6,6. However, it is easier to re-apply dye to Nylon 6. It takes a strong reaction to remove color from nylon compared to wool.
Alkali caused bleeding and the fugitive dye can relocate itself in a patterned carpet or be removed by cleaning.
Acidity such as from hydrochloric or sulfuric acid can also destroy dye and the dye sites. They can also melt the fiber.
ORP values of less than -300 or over 400 can destroy the color too. A mild reducer is the best neutralizer for a strong oxidizer, and a mild oxidizer is the best neutralizer for a strong reducer. Mixing strong reducers and oxidizers together on the carpet will create a lot of heat and poisonous gas.
Wool
Wool fiber cannot be harmed by strong acids, but its dyes can be affected. However, wool bleeds at slightly acidic pH values. Most wool will measure pH 5.5. That is because itis anionic, and its polarity starts to flip when its reading climbs over 5.9.
Summary
Once again, color loss is common to both wool and nylon carpet and could be either natural or chemical. Meter will help you determine which is which and will help you find a way to neutralize the problem so that new dye can be re-applied.
James¬? Jim” B. Smith is an IICRC-approved instructor and a senior practicing inspector and part of the voting consensus of the IICRCS1OOcleaning stan¬?dard. His educational studies come from Texas A&M University and the University of Houston. He has been in the cleaning industry since 1975. For more information, visit his website atwww.carpetinspector.com/jbs, call (972) 334-0533 or (800) 675-4003, or email[email protected].












